|
|
 Re: Physics question for non-Brits
|
Joined: Sep 2007
Posts: 820
3/4 Throttle
|

3/4 Throttle
Joined: Sep 2007
Posts: 820 |
Quote:
OK. Son woke up (finally).
It has to do with "super chilling" and pressure differentials.
That is, the temperature of the liquid is below freezing, but it is static so hasn't frozen yet. opening it changes the pressure and/or surface tension so it freezes. At just the right temperature, you could make it solid just by shaking it.
Kind of like the opposite effect of super heating your coffee in the microwave and it explodes when you put a spoon in it, disrupting the surface tension.
Phase diagram for water:
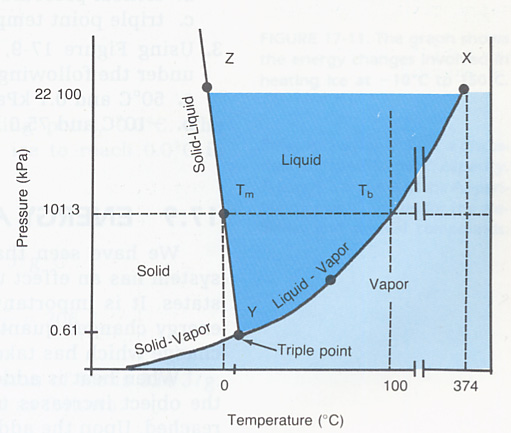
Are those double lines at about 200c to do with the surface tension/explosion thing?
Nice tasting ale at low strengths = longer drinking time & no fall on face flatty  
|
|
|
|
|
|
|